It is the policy of The University of Alabama to have all work involving biological material as well as all work involving recombinant or synthetic DNA reviewed by the Institutional Biosafety Committee (IBC). Approval must be granted prior to beginning any non-exempt work.
The approval of any BUA will be predicated on the following:
- A compliance-based lab inspection during the past year.
- A facility inspection and risk assessment report from the Biosafety Officer submitted to the IBC for review.
- The Principal Investigator, students, and staff have completed all safety training.
- If appropriate, all at-risk staff and students have been offered the Hepatitis B vaccination and received Bloodborne Pathogens training.
- All sections of the BUA form have been satisfactorily completed. Incomplete submissions will be sent back by OREC.
- All permits and Material Transfer Agreements (MTAs) have been completed, submitted to ORTA, and attached to BUA submission.
Documentation – Project Registration
Review guidance prior to completion of SOP Template.
Complete to submit.
Complete to submit a project registration to OREC.
Complete to submit a Minor Amendment to an existing IBC protocol (OREC).
Biological Use Authorization Timeline
BUA Timeline
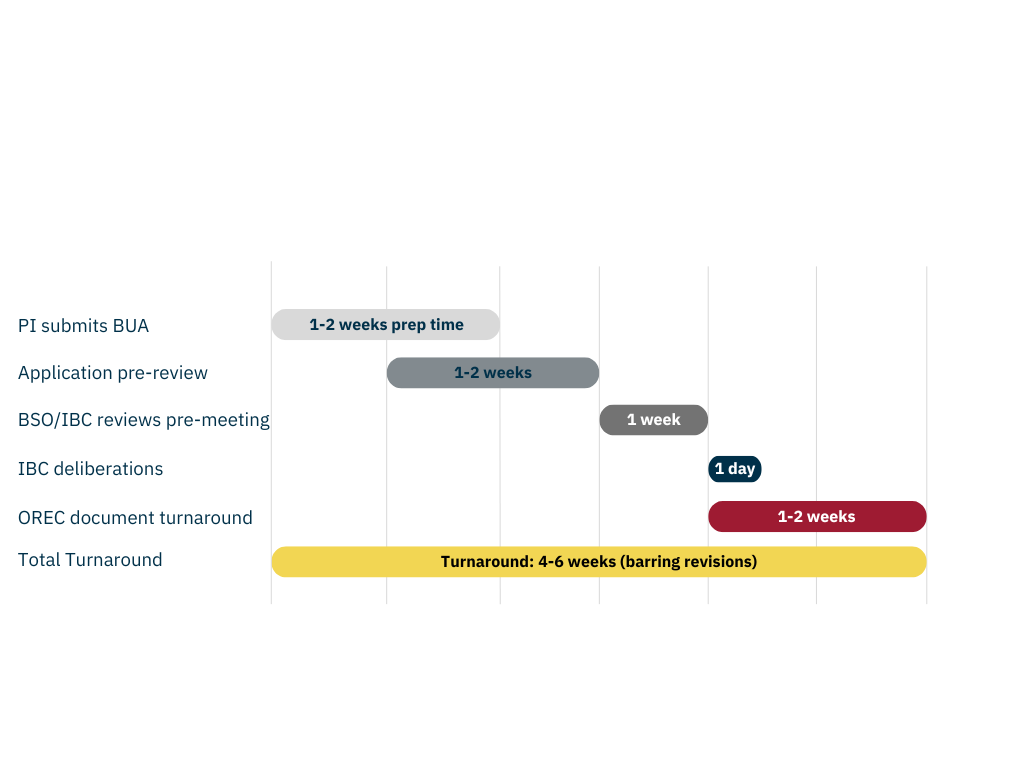
- PI submits BUA to OREC: 1-2 weeks prep time
- BSP/OREC reviews application: 1-2 weeks
- BSO/IBC reviews pre-meeting: 1 week
- IBC deliberations: 1 day
- OREC document turnaround: 1-2 weeks

A Project Registration via BUA is required for any research activity that may pose risks to humans, animals, plants, the environment, and the community at large. Registration includes both teaching and research activities.
A. Recombinant and synthetic DNA/RNA research (including exempt work*):
- genetic engineering and/or modification in any organism
- work with, or creation of, transgenic animals (vertebrates AND invertebrates), or transgenic plants
- synthetic biology
- any clinical trials involving viral vectors or human gene therapy
B. The use of risk groups RG1 and RG2 biohazardous agents:
- infectious agents: e.g., bacteria, viruses, prions, protozoa, fungi, etc.
- biologically derived toxins or select agents
- Human-derived materials: bloodborne pathogens (BBP) or other potentially infectious material (OPIM)
- work with human and animal cells or tissues in culture
C. Research with environmental samples, including soil and water
D. Work with animal-derived materials from:
- non-human primates
- ruminants or swine
- chickens or other fowl
- any wild vertebrate animals
E. Potentially pathogenic materials/organisms, including those that are considered hazardous to humans, animals, agriculture, and the environment
* Section III-F of the NIH Guidelines set forth eight categories of research that are exempt from the requirements of the NIH Guidelines; however, the University of Alabama still requires that Principal Investigators register these categories of work with the IBC by submitting an IBC application.
Application Review
Each Submission
Submissions must be received by the 1st of the month to be considered for the next IBC meeting. Unless otherwise stated, these IBC meetings occur on the first Tuesday of the month. BUA must be complete, including supporting documentation, permits, and SOPs. If the submission is complete and deemed fully eligible for IBC review, it will be posted to the next meeting's agenda.
INCOMPLETE SUBMISSIONS WILL BE RETURNED BY OREC TO THE APPLICANT. Questions and clarifications will be addressed with the PI.
The BSO reviews for the following:
- The Lab Registration and assigned Risk Group and Biosafety Containment Levels.
- SOPs for accurate risk assessment and risk mitigation (e.g., Transport or shipping of samples, DNA extraction procedure).
- Appropriate Risk Group (RG) and containment level (BSL).
- Potential for additional containment practices.
- Performance of a full inspection of lab space and equipment.
- Evaluation of the lab personnel experience and training (Skillsoft and CITI).
- Inspection results (most recent).
- The project abstract will be reviewed for continuity with procedural SOPs. It should be clear, concise, organized and appropriate for the intended audience (NOTE: Non-scientific community members also sit on the IBC.)
- Current Exposure Control Plan (for work with Bloodborne Pathogens and OPIM) as applicable.
- Proper waste disposal procedures.
Biohazardous Waste Handling
EHS Environmental and Lab Safety have a comprehensive management program to address the use, handling, storage and disposal of hazardous materials, including biological waste.
Additional Information and FAQs
For more detailed information about Project Registration and the BUA process: research.ua.edu